
Saluda Medical
Founded Year
2011Stage
Series G - II | AliveTotal Raised
$502.65MLast Raised
$100M | 8 mos agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
+33 points in the past 30 days
About Saluda Medical
Saluda Medical focuses on neuromodulation therapies within the healthcare sector, specializing in advanced closed-loop technologies for treating neurological disorders. The company's flagship product, the Evoke System, is a spinal cord stimulation device that utilizes ECAP-controlled closed-loop technology to manage chronic intractable pain, including conditions associated with failed back surgery syndrome and various types of leg and back pain. The Evoke System has been subjected to clinical trials. It was founded in 2011 and is based in Minneapolis, Minnesota.
Loading...
Loading...
Research containing Saluda Medical
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Saluda Medical in 2 CB Insights research briefs, most recently on Apr 17, 2025.
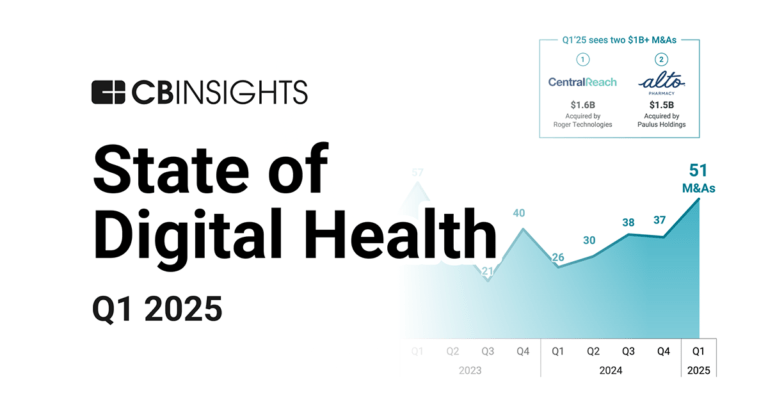
Apr 17, 2025 report
State of Digital Health Q1’25 Report
Jul 26, 2023 report
State of Digital Health Q2’23 ReportExpert Collections containing Saluda Medical
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Saluda Medical is included in 1 Expert Collection, including Digital Health.
Digital Health
11,440 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma, sequencing instruments, gene editing, and assistive tech.
Saluda Medical Patents
Saluda Medical has filed 139 patents.
The 3 most popular patent topics include:
- neurophysiology
- neurotechnology
- implants (medicine)
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
6/10/2024 | 3/25/2025 | Neurophysiology, Neurotechnology, Implants (medicine), Electrophysiology, Neurological disorders | Grant |
Application Date | 6/10/2024 |
|---|---|
Grant Date | 3/25/2025 |
Title | |
Related Topics | Neurophysiology, Neurotechnology, Implants (medicine), Electrophysiology, Neurological disorders |
Status | Grant |
Latest Saluda Medical News
Jul 19, 2025
EVA represents an extension of the Evoke System, which is Saluda Medical's inaugural product. July 18, 2025 The sensing technology is intended for scanning and analysing the spinal cord of individuals to offer personalised therapy. Saluda Medical has announced the full US commercial launch of its EVA sensing technology, which is intended for scanning and analysing the spinal cord of individuals to offer personalised therapy. In December 2024, the Food and Drug Administration (FDA) approved EVA, which is now compatible with all patients implanted with the Evoke SmartLoop System. Go deeper with GlobalData EVA represents an extension of the Evoke System, which is Saluda Medical’s inaugural product, indicated for managing chronic intractable pain. This includes unilateral or bilateral pain related to failed back surgery syndrome and intractable low back and leg pain. Saluda Medical chief commercial officer Mike Mathias said: “This full commercial launch represents a significant advancement in SCS therapy and delivers on the promise of objective dosing and more effective pain relief for patients. “In addition to its clinical benefits, EVA automates manual programming steps, thereby improving the patient experience. Since receiving FDA approval at the end of last year, EVA has been utilised in over 3,000 commercial patient visits through a limited market release.” The company noted that the Evoke SmartLoop System is known for optimising the outcomes of individuals by sensing, measuring, and adjusting stimulation based on the neural response biomarker of the patient, ensuring therapy is maintained at the prescribed level. GlobalData Strategic Intelligence US Tariffs are shifting - will you react or anticipate? Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis. By GlobalData The presentation of new clinical data on EVA will take place at the ASPN 2025 Annual Conference on 17-20 July 2025 in Florida, US. This conference will feature 11 abstracts and an oral presentation, contributing to the company’s clinical evidence, which includes more than 37 publications. The landmark EVOKE Study, which will be among the data presented, has shown long-term efficacy of up to 36 months. Saluda Medical develops treatments for chronic neurological conditions through its neuromodulation platform. Earlier this year, the company completed a financing round of $100m to further commercialise the Evoke System. Sign up for our daily news round-up! Give your business an edge with our leading industry insights.
Saluda Medical Frequently Asked Questions (FAQ)
When was Saluda Medical founded?
Saluda Medical was founded in 2011.
Where is Saluda Medical's headquarters?
Saluda Medical's headquarters is located at 9401 James Avenue South, Minneapolis.
What is Saluda Medical's latest funding round?
Saluda Medical's latest funding round is Series G - II.
How much did Saluda Medical raise?
Saluda Medical raised a total of $502.65M.
Who are the investors of Saluda Medical?
Investors of Saluda Medical include Redmile Group, Action Potential Venture Capital, Fidelity Investments, T. Rowe Price, Wellington Management and 9 more.
Who are Saluda Medical's competitors?
Competitors of Saluda Medical include Nevro.
Loading...
Compare Saluda Medical to Competitors
Micron Medical is involved in the development of investigational medical devices within the healthcare sector. The company provides neuromodulation devices for treating overactive bladder symptoms with minimally invasive procedures. Micron Medical serves the healthcare industry, including clinical trial participants and medical professionals. Micron Medical was formerly known as StimGuard. It was founded in 2019 and is based in Boca Raton, Florida.

SPR Therapeutics specializes in pain management solutions within the medical device industry. The company offers the SPRINT PNS System, a non-permanent, minimally invasive treatment designed to provide long-term relief from chronic and acute pain through peripheral nerve stimulation. It primarily serves the healthcare sector, offering solutions to both physicians and patients dealing with pain management. It was founded in 2010 and is based in Cleveland, Ohio.

Mainstay Medical focuses on innovative medical device solutions in the healthcare sector. The company offers a restorative neurostimulation therapy called ReActiv8, designed to treat mechanical chronic low back pain by stimulating the muscles that support the lumbar spine. This therapy aims to provide a non-invasive solution to improve stability and reduce pain for patients suffering from persistent back discomfort. It was founded in 2008 and is based in San Diego, California.
Bioness offers medical devices designed to benefit people with stroke, multiple sclerosis, traumatic brain injury, cerebral palsy, and spinal cord injury. Its products use electrical stimulation to help people regain mobility and independence and to improve quality of life and productivity. The company was founded in 1992 and is based in Valencia, California.

ReFlow Medical develops medical devices for the treatment of cardiovascular diseases within the healthcare industry. The company provides products for vascular interventions, including devices for treating peripheral and coronary artery conditions. ReFlow Medical serves the healthcare sector, focusing on medical professionals and hospitals. It is based in San Clemente, California.

NeuroSigma operates as a bioelectronics company that focuses on developing medical devices for neurological and neuropsychiatric disorders. Its main product, the Monarch eTNS System, is a non-invasive device designed to treat pediatric Attention Deficit Hyperactivity Disorder (ADHD) through external trigeminal nerve stimulation. It is primarily used in the home setting under caregiver supervision during sleep. It was founded in 2008 and is based in Los Angeles, California.
Loading...
